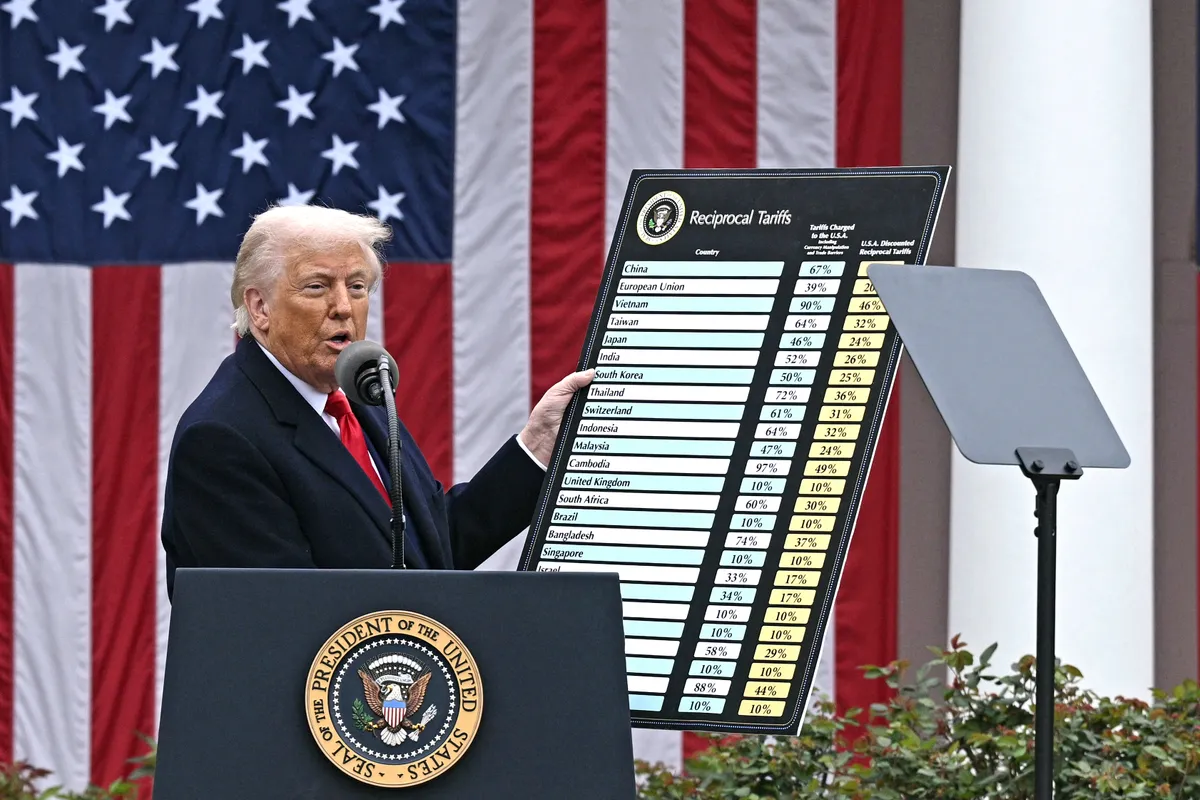
In recent years, personal protective equipment (PPE) has become one of Vietnam’s strategic export commodities, especially to the U.S. market. However, the global trade context is witnessing complex fluctuations — notably the high tariff policies imposed by the U.S. on goods imported from Asia, including Vietnam.
How can Vietnamese enterprises maintain competitiveness, adapt flexibly, and seize opportunities in such a challenging environment? The answer lies in building a smart, methodical, and internationally standardized export strategy.
Context: U.S. tightens supply chains and increases tariffs
At the end of 2024, the U.S. government announced an increase in tariffs on certain PPE products imported from Vietnam to as high as 46%, citing the need to safeguard supply chain security and reduce dependency on certain countries. This had an immediate impact on thousands of PPE shipments that were being produced and prepared for export.
In addition to tariffs, the U.S. has strengthened origin traceability requirements, demanding complete transparency regarding materials, manufacturing chains, and inspection standards. This is a key obstacle that has caused confusion for many Vietnamese PPE businesses during customs clearance in the U.S.
Strategic Response: Solutions for Vietnamese PPE Enterprises
Transparency in origin and trade documentation
The U.S. is placing special emphasis on the “Made in Vietnam” label. Therefore, businesses must:
- Ensure raw materials do not originate from countries on the U.S. watchlist.
- Maintain complete documentation from material sourcing, production, packaging, to shipment.
- Update and comply with the latest requirements for the Certificate of Origin (CO) as mandated by U.S. Customs and Border Protection (CBP).
Standardizing PPE Products to International Norms: From Mandatory Compliance to Long-Term Growth Strategy
✦ Why is international standardization essential when exporting PPE?
The U.S. is one of the most demanding markets globally. Authorities do not only inspect the appearance of shipments but also evaluate the entire production process, quality control capabilities, and risk management systems of suppliers. Therefore, international standardization is no longer optional — it is a prerequisite for Vietnamese PPE products to be accepted, remain, and grow in this market.
When PPE products such as masks, protective gear, and medical gloves are manufactured according to ISO 9001 and ISO 13485 standards, it proves that:
✅ The business has effective control over its entire production chain.
✅ Product quality is stable, traceable, and transparent.
✅ There is a ready system for issue response, product recall, and continuous improvement.
✦ ISO 9001: Foundation for Professional Quality Management
ISO 9001:2015 is the most widely adopted quality management standard in the manufacturing industry. PPE manufacturers applying ISO 9001 must:
- Establish standardized production procedures with quality control at each stage.
- Manage risks and opportunities in the supply chain (e.g., material fluctuation, technical incidents).
- Continuously improve based on real data (PDCA – Plan, Do, Check, Act).
- Train staff and clearly define roles and responsibilities.
💡 Specific Benefit:
When audited by U.S. importers, businesses with ISO 9001 systems can easily demonstrate transparent processes, thereby shortening customs clearance times and reducing the risk of shipment rejection.
✦ ISO 13485: The “Gold Standard” for Medical PPE Exported to the U.S.
If your business produces PPE for medical use — such as surgical masks, examination gloves, or isolation gowns — then ISO 13485:2016 is a required standard to:
- Supply products to hospitals and clinics in the U.S. and EU.
- File product registration with authorities such as the FDA (U.S.) or obtain CE Mark (EU) certification.
- Be selected by international distributors as a trusted supplier.
ISO 13485 has stricter requirements than ISO 9001, including:
- Managing product records throughout the lifecycle, from design to warranty and recall.
- A quick-response system for customer complaints.
- Hygiene control, sterilization, and production environment management (for medical products).
- Mandatory periodic inspections and ongoing risk assessments.
💡 Specific Benefit:
Being ISO 13485-certified allows businesses to pre-qualify for supplying PPE to major U.S. healthcare systems, which have rigorous legal and quality requirements.
Diversifying Export Markets and Distribution Channels: A Risk Reduction and Market Expansion Strategy
As the U.S. tightens tariff policies and import standards for PPE, relying solely on one market puts businesses in a passive position, especially amid geopolitical, legal, or currency fluctuations.
✦ Why diversify markets?
- Concentration Risk: If 70–90% of export revenue depends on the U.S., even minor legal or tariff changes can disrupt cash flow, cause inventory build-up, and result in customer loss.
- Intense Competition: The U.S. is a primary destination for PPE from China, India, Malaysia… leading to diminishing margins without competitive differentiation.
- Protectionist Trends: Post-COVID-19, the U.S. increasingly prioritizes domestic goods or those from bilateral FTA partners with binding conditions.
✦ Strategic Directions for Market Diversification
- Penetrate High-Standard but Stable Markets
- Europe (EU): Countries like Germany, France, and the Netherlands have high demand for medical and industrial PPE. The EVFTA allows many Vietnamese PPE products to enjoy duty exemptions or significant reductions.
- Japan & South Korea: Technically demanding but transparent and policy-stable markets. High-quality PPE may fetch 1.5–2x the prices of the U.S.
- Middle East: Countries like the UAE and Saudi Arabia are investing heavily in healthcare post-pandemic. These are niche, high-potential markets with PPE supply shortages.
👉 Note: To export effectively to these markets, businesses must:
- Have CE Marking (EU) or meet JIS standards (Japan).
- Localize packaging and technical documentation.
- Understand consumer culture and appropriate distribution channels.
- Diversify Distribution Channels in the U.S.
Besides market expansion, businesses should localize risk within the U.S. rather than only relying on FOB/CIF exports.
- Partner with domestic U.S. distributors: This shortens delivery time to end-users, optimizes warehousing and logistics costs, and ensures local representation to handle legal or warranty issues.
- Establish authorized agents or representative offices in key states such as California, Texas, and New York — where large Vietnamese communities and high PPE demand exist.
- Sell via international B2B platforms such as Amazon Business, Alibaba US, ThomasNet, or procurement systems for hospitals and government contractors.
💡 Specific Benefits:
- Adapt quickly to changing U.S. tariff regimes.
- Better market insight to tailor products to demand.
- Higher order conversion rates and shorter payment cycles.
Partnering with Professional Supply Chain Stakeholders: Optimizing from Production to Market Entry
Exporting PPE is no longer just about manufacturing and shipping. It’s an integrated challenge involving legal compliance, testing, logistics, certification, finance, and insurance. Going it alone can cause bottlenecks at any stage, leading to major losses.
✦ Essential Partners for Exporting PPE Successfully
- Legal & International Trade Partners
These professionals understand U.S. customs law, origin rules (CBP), trade tariffs, and medical product regulations.
They help businesses:
- Identify the correct HS code to avoid incorrect, high tax rates.
- Advise on safe contract structures to reduce payment and litigation risks.
- Guide self-certification documentation for FTAs or post-entry audits.
- PPE-Specialized Logistics Providers
Not all carriers understand PPE specifics. Some products require medical certifications, others must be kept in special conditions (temperature, humidity).
They offer:
- Expertise in customs declarations tailored to PPE at U.S. ports.
- Relationships with reliable customs brokers.
- “Door-to-door with insurance” services — essential for large shipments.
- Manufacturing, Inspection, and Certification Partners
Especially important when outsourcing or needing ISO, CE, GMP certification…
They help:
- Shorten the path to certification.
- Standardize quality systems per target markets.
- Protect your brand in the event of end-user disputes.
TM&DV 8688 COMPANY LIMITED – A Professional ISO-Certified PPE Export Partner
TM&DV 8688 COMPANY LIMITED is one of Vietnam’s pioneers in providing comprehensive PPE solutions to international standards, especially for the U.S. and European markets.
How 8688 Supports PPE Exporters:
✅ ISO 9001 & ISO 13485-certified products: All production processes operate under internationally recognized quality systems, ensuring product consistency and global inspection compliance.
✅ Full-package export consultancy: From technical advice, origin tracing, and customs declarations to logistics, 8688 supports businesses step-by-step to optimize cost and time.
✅ Transparent, modern supply chain: 8688 operates GMP-standard factories capable of fulfilling large, urgent, and high-quality orders.
“With practical experience in PPE export markets, we at 8688 not only supply products — we partner with you as an international trade expert.”
Conclusion: Turning Challenges into Growth Drivers
Change is inevitable in the global business environment. With shifting tariffs, quality standards, and origin regulations, PPE exporters must shift from short-term thinking to long-term strategic planning.
Investing in ISO-certified quality management, supply chain transparency, and collaborating with expert partners like TM&DV 8688 COMPANY LIMITED is the “key” to not only surviving but also thriving in demanding international markets like the United States.